Viewing posts for the category Featured on frontpage
Matching coloured LED combinations to a spectrum
Posted by: christian in Featured on frontpage on 26 Dec 2020
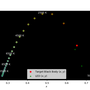
A previous blog post provides a class, ColourSystem, which can be used to predict the colour (within some colour system) of a provided spectrum. This post uses the class to determine how to combine a number of light emitting diodes (LEDs), or other light sources with known spectra in order to produce light with a given spectrum. This task is not as simple as fitting a linear combination of the LED spectra to the given spectrum because the colour matching functions determining the tristimulus values (which, in turn model the colour perceived by the human eye) vary with wavelength and overlap. Also, LEDs emit light, so no negative coefficient in such a linear combination can be allowed.
Animated contour plots with Matplotlib
Posted by: christian in Featured on frontpage on 12 Dec 2020
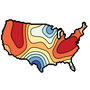
A quick project inspired by this tweet by @story645 referencing Jacques Bertin's Semiology of Graphics.
Uranium enrichment and the separative work unit (SWU)
Posted by: christian in Featured on frontpage on 30 Nov 2020

Natural uranium consists largely of two isotopes, $\mathrm{^{235}U}$ and $\mathrm{^{238}U}$. The less-abundant (0.72%) isotope, $\mathrm{^{235}U}$ , is important for nuclear reactors and weapons because it is the only isotope existing in nature to any appreciable extent that can sustain a fission chain reaction (that is, it is fissile).
Visualizing vibronic transitions in a diatomic molecule
Posted by: christian in Featured on frontpage on 7 Oct 2020

The Morse oscillator code introduced in a previous blog post can be used to visualize the vibronic transitions in a diatomic molecule by creating two Morse objects (one for each electronic state) and plotting their potential energy curves and energy levels on the same Matplotlib Axes object.
Plotting nuclide halflives
Posted by: christian in Featured on frontpage on 29 Sep 2020

More than 3000 nuclides (atomic species characterised by the number of neutrons and protons in their nuclei) are known, most of them radioactive with a half-life of less than an hour. About 250 or so of them are stable (not observed to decay using presently-available instruments). The IAEA has an interactive online browser of the nuclides.