The harmonic oscillator wavefunctions
Posted on 11 May 2019
The harmonic oscillator is often used as an approximate model for the behaviour of some quantum systems, for example the vibrations of a diatomic molecule. The Schrödinger equation for a particle of mass $m$ moving in one dimension in a potential $V(x) = \frac{1}{2}kx^2$ is $$ -\frac{\hbar^2}{2m}\frac{\mathrm{d}^2\psi}{\mathrm{d}x^2} + \frac{1}{2}kx^2\psi = E\psi. $$ With the change of variable, $q = (mk/\hbar^2)^{1/4}x$, this equation becomes $$ -\frac{1}{2}\frac{\mathrm{d}^2\psi}{\mathrm{d}q^2} + \frac{1}{2}q^2\psi = \frac{E}{\hbar\omega}\psi, $$ where $\omega = \sqrt{k/m}$. This differential equation has an exact solution in terms of a quantum number $v=0,1,2,\cdots$: $$ \psi(q) = N_vH_v(q)\exp(-q^2/2), $$ where $N_v = (\sqrt{\pi}2^vv! )^{-1/2}$ is a normalization constant and $H_v(q)$ is the Hermite polynomial of order $v$, defined by: $$ H_v(q) = (-1)^ve^{q^2}\frac{\mathrm{d}^v}{\mathrm{d}q^v}\left(e^{-q^2}\right). $$ The Hermite polynomials obey a useful recursion formula: $$ H_{n+1}(q) = 2qH_n(q) - 2nH_{n-1}(q), $$ so given the first two: $H_0 = 1$ and $H_1 = 2q$, we can calculate all the others.
The following code plots the harmonic oscillator wavefunctions (PLOT_PROB = False) or probability densities (PLOT_PROB = True) for vibrational levels up to VMAX on the vibrational energy levels depicted within the potential, $V = q^2/2$.
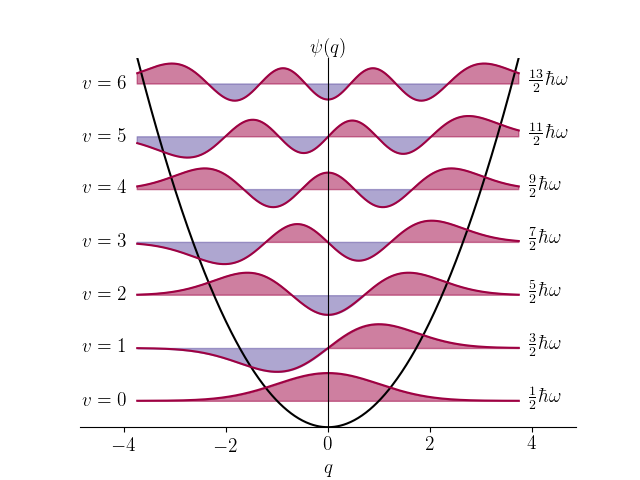
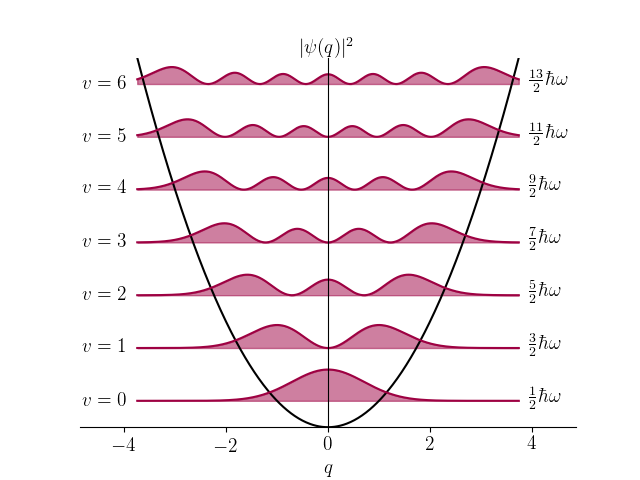
import numpy as np
from matplotlib import rc
from scipy.special import factorial
import matplotlib.pyplot as plt
rc('font', **{'family': 'serif', 'serif': ['Computer Modern'], 'size': 14})
rc('text', usetex=True)
# PLOT_PROB=False plots the wavefunction, psi; PLOT_PROB=True plots |psi|^2
PLOT_PROB = False
# Maximum vibrational quantum number to calculate wavefunction for
VMAX = 6
# Some appearance settings
# Pad the q-axis on each side of the maximum turning points by this fraction
QPAD_FRAC = 1.3
# Scale the wavefunctions by this much so they don't overlap
SCALING = 0.7
# Colours of the positive and negative parts of the wavefunction
COLOUR1 = (0.6196, 0.0039, 0.2588, 1.0)
COLOUR2 = (0.3686, 0.3098, 0.6353, 1.0)
# Normalization constant and energy for vibrational state v
N = lambda v: 1./np.sqrt(np.sqrt(np.pi)*2**v*factorial(v))
get_E = lambda v: v + 0.5
def make_Hr():
"""Return a list of np.poly1d objects representing Hermite polynomials."""
# Define the Hermite polynomials up to order VMAX by recursion:
# H_[v] = 2qH_[v-1] - 2(v-1)H_[v-2]
Hr = [None] * (VMAX + 1)
Hr[0] = np.poly1d([1.,])
Hr[1] = np.poly1d([2., 0.])
for v in range(2, VMAX+1):
Hr[v] = Hr[1]*Hr[v-1] - 2*(v-1)*Hr[v-2]
return Hr
Hr = make_Hr()
def get_psi(v, q):
"""Return the harmonic oscillator wavefunction for level v on grid q."""
return N(v)*Hr[v](q)*np.exp(-q*q/2.)
def get_turning_points(v):
"""Return the classical turning points for state v."""
qmax = np.sqrt(2. * get_E(v + 0.5))
return -qmax, qmax
def get_potential(q):
"""Return potential energy on scaled oscillator displacement grid q."""
return q**2 / 2
fig, ax = plt.subplots()
qmin, qmax = get_turning_points(VMAX)
xmin, xmax = QPAD_FRAC * qmin, QPAD_FRAC * qmax
q = np.linspace(qmin, qmax, 500)
V = get_potential(q)
def plot_func(ax, f, scaling=1, yoffset=0):
"""Plot f*scaling with offset yoffset.
The curve above the offset is filled with COLOUR1; the curve below is
filled with COLOUR2.
"""
ax.plot(q, f*scaling + yoffset, color=COLOUR1)
ax.fill_between(q, f*scaling + yoffset, yoffset, f > 0.,
color=COLOUR1, alpha=0.5)
ax.fill_between(q, f*scaling + yoffset, yoffset, f < 0.,
color=COLOUR2, alpha=0.5)
# Plot the potential, V(q).
ax.plot(q, V, color='k', linewidth=1.5)
# Plot each of the wavefunctions (or probability distributions) up to VMAX.
for v in range(VMAX+1):
psi_v = get_psi(v, q)
E_v = get_E(v)
if PLOT_PROB:
plot_func(ax, psi_v**2, scaling=SCALING*1.5, yoffset=E_v)
else:
plot_func(ax, psi_v, scaling=SCALING, yoffset=E_v)
# The energy, E = (v+0.5).hbar.omega.
ax.text(s=r'$\frac{{{}}}{{2}}\hbar\omega$'.format(2*v+1), x=qmax+0.2,
y=E_v, va='center')
# Label the vibrational levels.
ax.text(s=r'$v={}$'.format(v), x=qmin-0.2, y=E_v, va='center', ha='right')
# The top of the plot, plus a bit.
ymax = E_v+0.5
if PLOT_PROB:
ylabel = r'$|\psi(q)|^2$'
else:
ylabel = r'$\psi(q)$'
ax.text(s=ylabel, x=0, y=ymax, va='bottom', ha='center')
ax.set_xlabel('$q$')
ax.set_xlim(xmin, xmax)
ax.set_ylim(0, ymax)
ax.spines['left'].set_position('center')
ax.set_yticks([])
ax.spines['top'].set_visible(False)
ax.spines['right'].set_visible(False)
plt.savefig('sho-psi{}-{}.png'.format(PLOT_PROB+1, VMAX))
plt.show()